Stabilizing interactions
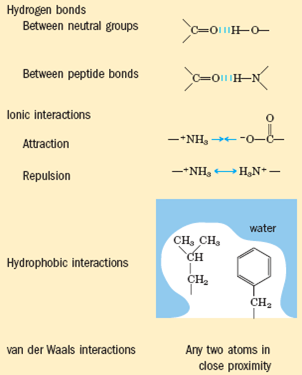
1) Hydrogen bonds: weak electrostatic attractions between one electronegative atom (such as oxygen or nitrogen) and a hydrogen atom covalently linked to a second electronegative atom.
(2) Electrostatic interactions: relatively weak charge-charge interactions (attractions of opposite charges, repulsions of like charges) between two ionized groups.
(3) Hydrophobic interactions: the forces that tend to bring two hydrophobic groups together, reducing the total area of the two groups that is exposed to surrounding molecules of the polar solvent (water).
(4) Van der Waals interactions: weak interactions between the electric dipoles that two close-spaced atoms induce in each other.

This site is one of the best!!!
ReplyDeletethis site is really very helpful...
ReplyDeletei thanks all who support students.v.best site
ReplyDeletevery helpful site
ReplyDeletevery helpful site....i would appreciate if you also provide sums based on pcr and molecular biology.
ReplyDeletevery informative site
ReplyDeleteitz really helpful...brief and to the point.
ReplyDeleteits very usefull for competitive exam preparation notes r very super to the point study ....good work
ReplyDeleteby amudha vasavi college erode
I love this site.. its helpful
ReplyDeleteThank you so much for your encouragement.
ReplyDeleteThanks...😊😊😊
ReplyDeleteThis site is really helpful...
More questions on ph
ReplyDeleteThis site is best....ur work is appreciable thanks.
ReplyDelete