What are Enzymes?
Enzymes are biological
catalysts that speed up the reaction rate by lowering activation energy without
undergoing any change by itself.
Nomenclature Committee of the International
Union of Biochemistry and Molecular Biology (NC-IUBMB) proposed a system for Classification
and Naming of Enzyme-Catalyzed Reactions.
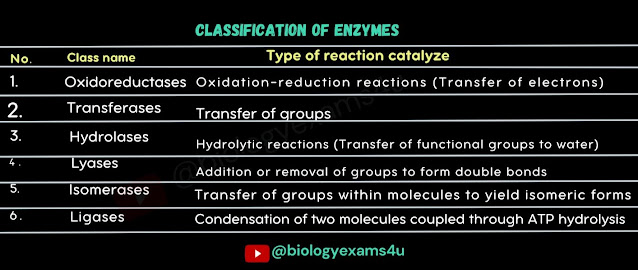
The 6 main classes are
- Class 1:
Oxidoreductases: all enzymes catalyzing
oxidoreduction reactions
- Class 2: transferases: Enzymes catalyzing transfer of
functional group from one compound to another
- Class 3: Hydrolases: Enzymes catalyzing the hydrolytic
cleavage of C-O, C-N, C-C and some other bonds.
- Class 4: Lyases: enzymes cleaving C-C, C-O, C-N, and
other bonds by forming double bonds or rings other than hydrolysis or oxidation
- Class 5: Isomerases: Enzymes catalyzing rearrangement of
atoms within a molecule
- Class 6: Ligases: catalyzing the joining together of
two molecules coupled with the hydrolysis of a diphosphate bond in ATP or a
similar triphosphate.
Class 1:
Oxidoreductases: all enzymes catalyzing
oxidoreduction reactions
Examples: Glucose oxidase,
Succinate dehydrogenase
Step 6 of Krebs cycle
Explanation of the reaction:
- Succinate dehydrogenase that catalyzes the reaction or oxidation of succinate to Fumarate.
- Hydrogen is donated by the succinate so it become oxidized this hydrogen is received by this fad that becomes fadh2 here fad is reduced to fadh2 a reduction reaction has happened.
- In this reaction, hydrogen is donated by succinate; that is succinate is oxidized to form the Fumarate so oxidation and reduction reaction is coupled and the enzyme is called dehydrogenase as hydrogen is removed from succinate.
- Oxidase is only used in cases where O2 is the acceptor.
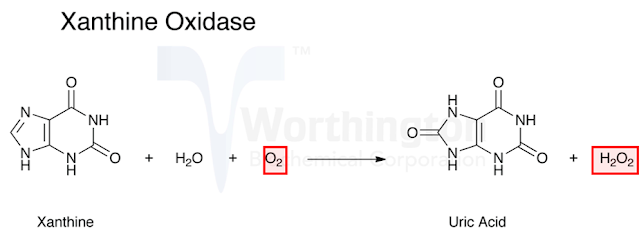
Case 2: Xanthine
oxidase:
xanthine oxidase catalyzes the oxidation of xanthine to form uric acid.
Here
oxygen is the acceptor therefore the term oxidase should be used as per the
nomenclature committee. So if an enzyme is named as oxidase it suggests that
oxygen is the acceptor and often H2O2 or H2O is produced in the reaction.
Other
examples: glucose oxidase, cytochrome oxidase, monoamine oxidase
Class 2: Transferases: Enzymes catalyzing transfer of functional
group from one compound to another.
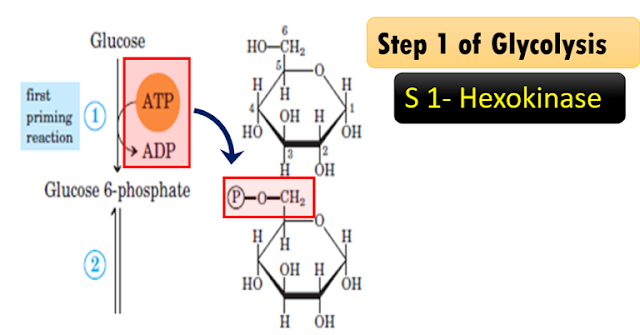
Step 1 of Glycolysis
Explanation of the
reaction: Hexokinase, a transferase enzyme
Glucose becomes
glucose 6-phosphate; catalyzed by enzyme hexokinase. Glucose receives a
phosphate from ATP; therefore, ATP becomes ADP and the phosphate is donated to
this glucose. Glucose becomes glucose 6-phosphate as a phosphate group is
transferred from one molecule; that is ATP to glucose forming glucose
6-phosphate. The enzyme is kinase. It is called as hexokinase as glucose is a six-carbon
compound.
Class 3: Hydrolases: Enzymes catalyzing the hydrolytic
cleavage
Protease
Explanation of the
reaction: Protease, a hydrolase enzyme
Proteases
catalyze hydrolytic reactions that degrade protein molecules down to
peptides and eventually to free amino acids. These class of enzymes often ends
with ’ase’.
Other examples: Lipase, Protease, Nuclease, Amylase, phosphatase.
Class 4: Lyases: enzymes cleaving C-C, C-O, C-N, and
other bonds by forming double bonds or rings other than hydrolysis or oxidation
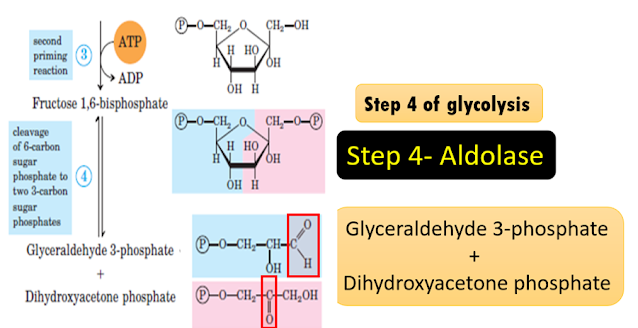
Step 4 of glycolysis
Explanation of the
reaction: Aldolase, a Lyase
In
glycolysis, the lyase called aldolase catalyses the readily reversible
splitting of fructose 1,6-bisphosphate (F-1,6-BP),into the products
glyceraldehyde 3-phosphate (GAP) and dihydroxyacetone phosphate (DHAP). This is
an example of a lyase that helps to cleave carbon-carbon bonds. The splitting
is not a hydrolysis or an oxidation. Here a double bond is formed as you see in
the aldehyde group of GAP and ketone group of DHAP.
Other
Examples: Citrate lyase, Isocitrate lyase, Pectate lyase
Class 5: Isomerases:
Enzymes catalyzing rearrangement of atoms within a molecule
Step 2 of Glycolysis
Explanation of the
reaction: Phosphoglucose Isomerase
Glucose
6-phosphate becomes fructose 6-phosphate, catalysed by the enzyme Phosphoglucose
Isomerase. The structural arrangement of atoms has changed. Glucose and
fructose are isomers with the same molecular formula C6H12O6
but differ in structure. Glucose is an aldose with aldehyde group and fructose
is a ketose with ketone group.
Other Examples:
- Triose phosphate isomerase
- Glucose isomerase
- Protein disulfide-isomerase
Class 6: Ligases: Joining Enzymes
Enzymes
joining together two molecules with the hydrolysis of a diphosphate bond in ATP
or a similar triphosphate.
Explanation of the
reaction: aminoacyl-tRNA synthetase in Protein translation
An aminoacyl-tRNA synthetase, also called tRNA-ligase, is an enzyme that attaches the appropriate amino acid onto its corresponding tRNA.
Here amino acid,
methionine is joined to its corresponding tRNA by the enzyme tRNA-ligase for
methionine along with the hydrolysis of one molecule of ATP, yielding
aminoacyl-tRNA (Charged tRNA-Met), AMP, and PPi.
Example 2
Glutamate cysteine ligase (GCL) catalyzes
the first and rate-limiting step in the production of the cellular antioxidant
glutathione (GSH). GCL joins Glutamate and cysteine to form Glutamyl Cysteine.
Other Examples:
- Ubiquitin Ligases (C-N bond)
- Glutamate–cysteine ligase (C-N bond)
- Aminoacyl tRNA synthetase (C-O bond)
- Succinyl coenzyme A synthetase (C-S bond)
- DNA ligase
These are the six major classes of enzymes. Thank you so much for your support.