Water (H2O) is an inorganic polar compound composed of molecules of hydrogen and oxygen. The most crucial property for all the other properties is the polarity of water.
7 properties of water
1.Water is polar.
To understand the importance of polarity, we must know the bonds
in water.
Which are the bonds in water?
Water molecule has two types of bonds:
Covalent Bond: Each water molecule is formed by two hydrogen atoms
covalently bonded to one oxygen atom. A covalent bond is formed when two atoms
share electrons. Unequal sharing of electrons makes water a polar molecule. The
oxygen end of the molecule is slightly negative and hydrogen end is slightly
positive.
Hydrogen bond: H-bonds are formed between water molecules due to
the polar nature of the water molecule. The hydrogen atom of one water molecule;
with partial positive charge is attracted to the oxygen atom of another water molecule;
carries a partial negative charge. This is due, where the oxygen atom and the
hydrogen atom carry a partial positive charge.
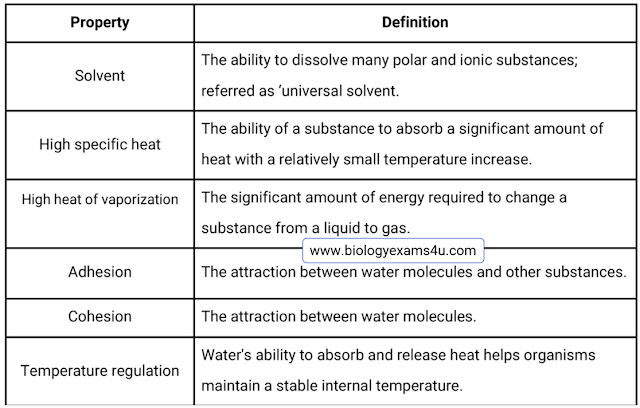
2. Water as Solvent:
It can dissolve more substances than any other liquid. That is why, Water is
called as the “universal solvent.” This property is crucial because it means
that wherever water goes, whether through the air, the ground, or through our
bodies, it carries along valuable chemicals, minerals, and nutrients. The
solvent property of water is primarily due to its polarity and ability to form
hydrogen bonds.
Image credit: https://bio.libretexts.org/
Example: when NaCl crystals are added to water, NaCl dissociate into Na+ and Cl– ions, and spheres of hydration form around the ions. The positively charged sodium ion is surrounded by the partially negative charge of the water molecule’s oxygen. The negatively charged chloride ion is surrounded by the partially positive charge of the hydrogen on the water molecule. This is called as a sphere of hydration, or hydration shell and serves to keep the particles dispersed in the water.
Importance of
water as solvent
- Medium for Chemical reactions: Water makes up about 70% of our cell. All
metabolic reactions essential for life takes place in a watery environment
inside our cell. This is due to the solvent property of water.
- Transportation
of Nutrients: Water’s ability to
dissolve various substances allows it to carry valuable chemicals, minerals,
and nutrients inside our body and also in the environment.
- Formation of
Solutions: Water can form solutions
with a wide range of substances like sugar solution and salt solution vital for
many biological and physical phenomena.
3.High
Specific Heat: The ability of water to
absorb a significant amount of heat with a relatively small temperature
increase. The property of water to
resist changes in temperature is known as its high specific heat capacity.
This
property of water plays a crucial role in maintaining the Earth’s climate.
Large bodies of water like oceans can absorb a lot of heat during the day
without their temperature rising significantly. At night, this heat is slowly
released, helping to moderate temperatures.
Let us
consider a simple example of water and a metal, like aluminum, under the sun.
Both water and aluminum receive the same amount of sunlight and thus absorb the
same amount of heat energy. However, you will notice that the aluminum gets hot
very quickly, while the water heats up much more slowly.
As the
temperature rises, the hydrogen bonds between water continually break and
reform, allowing for the overall temperature to remain stable, although
increased energy is
4.High Heat
of vaporization refers to the high amount of
energy required to change a substance from liquid to gas.
Example: When
you exercise, your body heats up. To cool down, your body produces sweat, which
is mostly water. As the sweat evaporates from your skin, it takes with it a
large amount of heat due to water’s high heat of vaporization. This is why you
feel cooler when your sweat evaporates.
This property
water plays a crucial role in many natural processes, including weather, climate,
and temperature regulation in organisms.
5 & 6. Adhesion
and Cohesion
Adhesion: The
ability of water to sticks to other surfaces. A simple example of adhesion is
capillary action, where water “climbs” upwards through thin glass tubes (called
capillary tubes) placed in a beaker of water. This upward motion against
gravity depends on the attraction between water molecules and the glass walls
of the tube (adhesion), as well as on interactions between water molecules
(cohesion). The same thing happens in water transport through xylem in plants.
Cohesion: The
property of water molecules to attract with each other, leading to phenomena
like surface tension. Water striders walking on water surface due to surface
tension.
- Visit our TPT store by clicking here.
- Download free resources or purchase worksheets on Properties of Water.
- Please rate the product and follow us on store.

